1. Introduction
2. Materials and Methods
2.1 Materials
2.2 Silane-modification of cellulose nanofiber powder
2.3 Preparation of bio-polyurethane/silane-modified cellulose nanofiber nanocomposites
2.4 Characterization of silane-modified cellulose nanofiber
2.5 Characterization of bio-polyurethane/silane-modified cellulose nanofiber nanocomposites
3. Results and Discussion
3.1 Hydrophobic expression of silanemodified cellulose nanofiber
3.2 Characterization of silane-modified cellulose nanofiber
3.3 Characterization of bio-urethane/silane-modified cellulose nanofiber composites
4. Conclusions
1. Introduction
The increased use of fossil fuel-derived products is a direct consequence of the Earth’s growing human population. To replace current petroleum-based items, research and development efforts are concentrating on the creation of more sustainable, renewable, and biodegradable materials. Cellulose, an abundant biological resource found in plants, animals, and microbes, can be processed into nanoscale materials known as nanocellulose.1,2) This includes three major groups: cellulose nanocrystals (CNC), cellulose nanofibers (CNF), and bacterial cellulose.3) In polymer composites, nanocellulose often serves as a strengthening component.4,5,6) CNFs, in particular, exhibit a more substantial enhancement impact compared to CNCs due to their improved miscibility. The characteristics of the composite are influenced by the compositions of the matrix and reinforcement materials, as well as the geometry of the reinforcement.7) CNF naturally forms entangled and hydrogen-bonded networks, imparting it with a unique ability to create robust films.8)
Moreover, owing to their ready availability, high strength-to-weight ratio, and cost-effectiveness, cellulose nanofibers are employed in this study for polyurethane composites as eco-friendly materials.9,10) Cellulose, possessing hydroxyl groups on its fiber surface, exhibits a hydrophilic nature in contrast to polyurethane. The polarity difference between these materials theoretically hinders their effective combination. Consequently, an additional step of surface modification of cellulose nanofibers can be introduced before composite production. Polyurethane (PU)-based composites have been extensively investigated and utilized in polymer and materials sciences due to their exceptional mechanical, gas barrier, and water barrier properties.11,12,13,14,15,16) Given their remarkable biocompatibility and biodegradability, they find diverse applications in tissue engineering and have significant industrial and medicinal uses. The synergistic combination of cellulose pulp and polyurethane has been shown to reduce dependence on synthetic resources to some extent. Furthermore, these plant-based natural fiber-reinforced polymer matrix composites promote the use of renewable resources.17,18,19,20,21)
Polyurethane and CNF are thermodynamically immiscible since PU is hydrophobic, and CNF is hydrophilic. To enhance the interaction between the matrix and reinforcement, both phases should share the same polarity.22,23) Insufficient adhesion between the two components leads to poor and inconsistent performance of the composite. Therefore, a surface modification procedure is employed to change the hydrophilicity of the reinforcement to hydrophobic, making it simple and cost-effective.
The hydrophilic surface of CNF can be transformed into a hydrophobic one using various chemical and physical adsorption techniques. However, chemical surface alterations are more reliable due to the formation of strong cross-links, including covalent bonds.24) Coupling agents and polymer drawing are two types of chemical surface modification. Surface modification using coupling agents can transfer the maximum strength from the matrix to the fiber.25) Silylation and acetylation are two commonly used coupling agents, with silylation being the focus of this research due to its ease, low cost, and widespread availability of chemicals compared to acetylation. The study utilizes MTMS for silylation, as it falls under the category of organic-inorganic coupling agents known as silanes.26) Silanes readily hydrolyze in aqueous solutions, forming silanol groups (Si-OH), facilitating their binding to hydroxyl-containing surfaces. This process breaks down the hydroxyl groups on the fiber surface, creating a Si-O-C link that changes the hydrophilic qualities of CNF to hydrophobic.27)
The research aims to compare modified and unmodified CNF in terms of wetting, chemical, thermal, morphological, and dispersion abilities. Additionally, it seeks to determine the effects of various contents of surface-modified CNF on polyurethane films.
2. Materials and Methods
2.1 Materials
In this study, CNF of powder type purchased from CNNT (South Korea) was employed as a material to impart characteristics. MTMS (Daejung, South Korea), a methyl-based silane compound, was chosen as the modifier to render cellulose nanofiber hydrophobic. Hydrochloric acid (2N HCl, Daejung, South Korea) and sodium hydroxide of beads type (NaOH, Daejung, South Korea) were utilized to adjust the pH during the silanization reaction. A bio-polyurethane (B-PU, BSG Co., Ltd., South Korea) with 44 wt.% biomass content was used to manufacture the nanocomposite film. Toluene (Daejung, South Korea) was utilized as a solvent to dilute B-PU. To analysis the zeta-potential, it is used the dimethylformamide (DMF, Daejung, South Korea).
2.2 Silane-modification of cellulose nanofiber powder
For CNF, the modification reaction was carried out without any specific pretreatment process since it disperses well in distilled water, which is a hydrophilic solvent. Firstly, to induce the hydrolysis reaction of MTMS, a certain amount of HCl and NaOH were added to 100 g of distilled water to create a solution with a pH 4. Then, 20 g of CNF was added, and the mixture was stirred at 500 rpm for 30 min. Subsequently, MTMS was gradually added to 200 g of distilled water at 20, 40, 80, and 160 g portions. The MTMS solution was stirred at 500 rpm for 10 min to prepare the MTMS solution. This MTMS solution was slowly added to the CNF solution, and the mixture was stirred at 500 rpm for 3 h to carry out silane modification on the surface of CNF. The silane-modified CNF (Si-CNF) was then dried and stored at room temperature.
2.3 Preparation of bio-polyurethane/silane-modified cellulose nanofiber nanocomposites
The B-PU resin has a high viscosity in the range of 40,000 to 60,000 cps at 25°C. To prepare the precursor solution, it was necessary to dilute the high-viscosity bio-polyurethane resin with a highly soluble solvent, such as toluene. Subsequently, the modified CNF was added to create solutions with 1, 2, 4, and 8 wt.% concentrations relative to the solid content of the B-PU resin. To ensure proper dispersion, a homo-mixer (T25D, IKA, Germany) was employed, and the mixture was stirred at 6,000 rpm for 20 min. Each concentration solution was then cast by drying at 110°C to evaporate the organic solvent and prepare the final films.
2.4 Characterization of silane-modified cellulose nanofiber
The contact angle for the weight ratios of Si-CNF to pure CNF at 1:1, 1:2, 1:4, and 1:8 was measured using a Drop Shape Analyzer from KRÜSS. A small volume of 4 μL was dropped, and contact angles were measured at 30, 60, 120, and 180 s, allowing observations of changes in contact angles over time. The contact angle variation can indicate the extent of modification, as it corresponds to changes in hydrophobicity. Zeta-potential values were measured using ASTM E2865-12 to assess the dispersion stability of Si-CNF in hydrophobic solvents. By comparing zeta-potential values between Si-CNF and pure CNF, the progress of hydrophobic modification could be inferred. An increasing absolute value of zeta-potential suggests better dispersion stability in the solvent. Evaluation of the modification status was also conducted through Fourier transform infrared (FT-IR, Perkin Elmer, Waltham, MA, USA) spectroscopy of Si-CNF and pure CNF. Fiber surfaces of both were observed using scanning electron microscope (SEM, SU-8220, HITACHI, Japan), and energy dispersive spectrometer (EDS) was employed to confirm the presence of Si elements, indicating successful modification. Additionally, thermal stability analysis was performed using thermogravimetric analyzer (TGA, TA instruments, USA). The heating rate was 10°C/min, reaching 800°C, and the analysis followed ASTM E1131 specifications.
2.5 Characterization of bio-polyurethane/silane-modified cellulose nanofiber nanocomposites
After incorporating the Si-CNF into the B-PU resin to manufacture films, the cross-section of the nanocomposite film was analyzed using SEM. Additionally, EDS analysis was conducted on both B-PU and B-PU/Si-CNF composite films to confirm the successful incorporation of Si-CNF into B-PU. The thermal stability of the B-PU/Si-CNF nanocomposite film was investigated using TGA. The FT-IR analysis covered the range of 600 to 4000 cm-1. X-ray diffraction (XRD, D/Max-2500, Rigaku, Tokyo, Japan) measurements were performed to examine the structure of the B-PU/Si-CNF nanocomposite film, focusing on the range of 10° to 50°. For mechanical characterization, films with a thickness of 0.05 mm or less were prepared according to ASTM D882 standards. Specimens, each measuring 15×90 mm2, were created for both polyurethane and B-PU/Si-CNF films. Five specimens were manufactured for each composition, and the tensile properties were determined using a universal testing machine (UTM, TXA, France).
3. Results and Discussion
3.1 Hydrophobic expression of silanemodified cellulose nanofiber
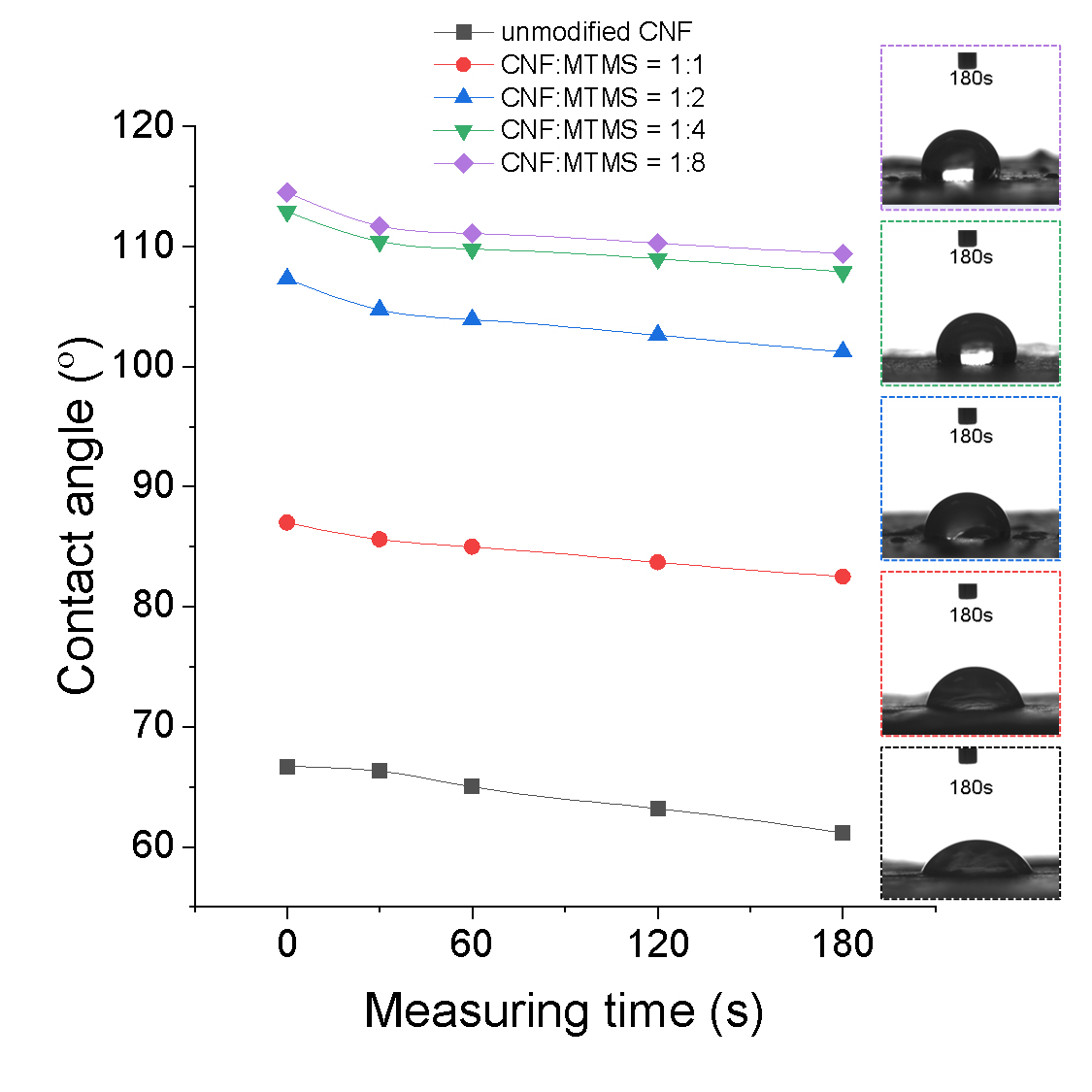
The contact angle was measured for pure CNF and CNF modified by silane at different weight ratios to the CNF: 1:1, 1:2, 1:4, and 1:8. The initial contact angle for pure CNF was 66.7°, and the contact angle at 180s decreased to 61.2°, indicating a gradual reduction over time. For Si-CNF at a ratio of 1:1, the initial contact angle was 87.0°, and after 180s, it measured 82.5°. CNFs with a 1:2 modification showed an initial contact angle of 107.3°, which decreased to 101.2° after 180s. CNFs with a 1:4 modification had an initial contact angle of 112.9°, and the contact angle after 180s was 107.9°. For CNFs with a 1:8 modification, the initial contact angle was 114.5°, and after 180s, it measured 109.4°.
The increase in MTMS content led to an increase in hydrophobicity, as evidenced by the rise in contact angle, as summarized in Fig. 1. Notably, the hydrophobicity showed a sharp increase when the MTMS content was increased to 1:2, but further increments did not lead to a significant rise. Therefore, considering economic and environmental factors, the ratio in this study was set at 1:2.
3.2 Characterization of silane-modified cellulose nanofiber
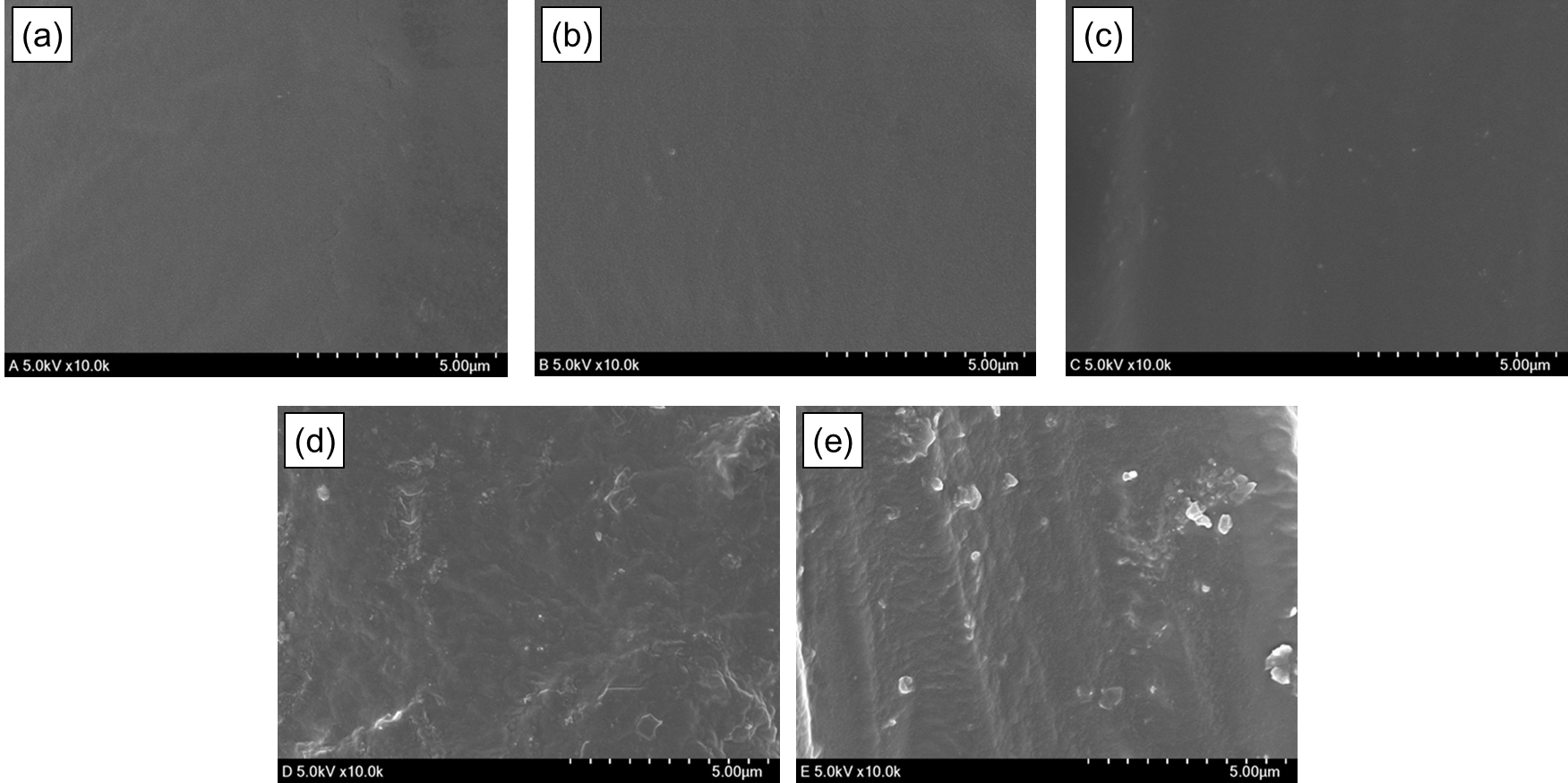
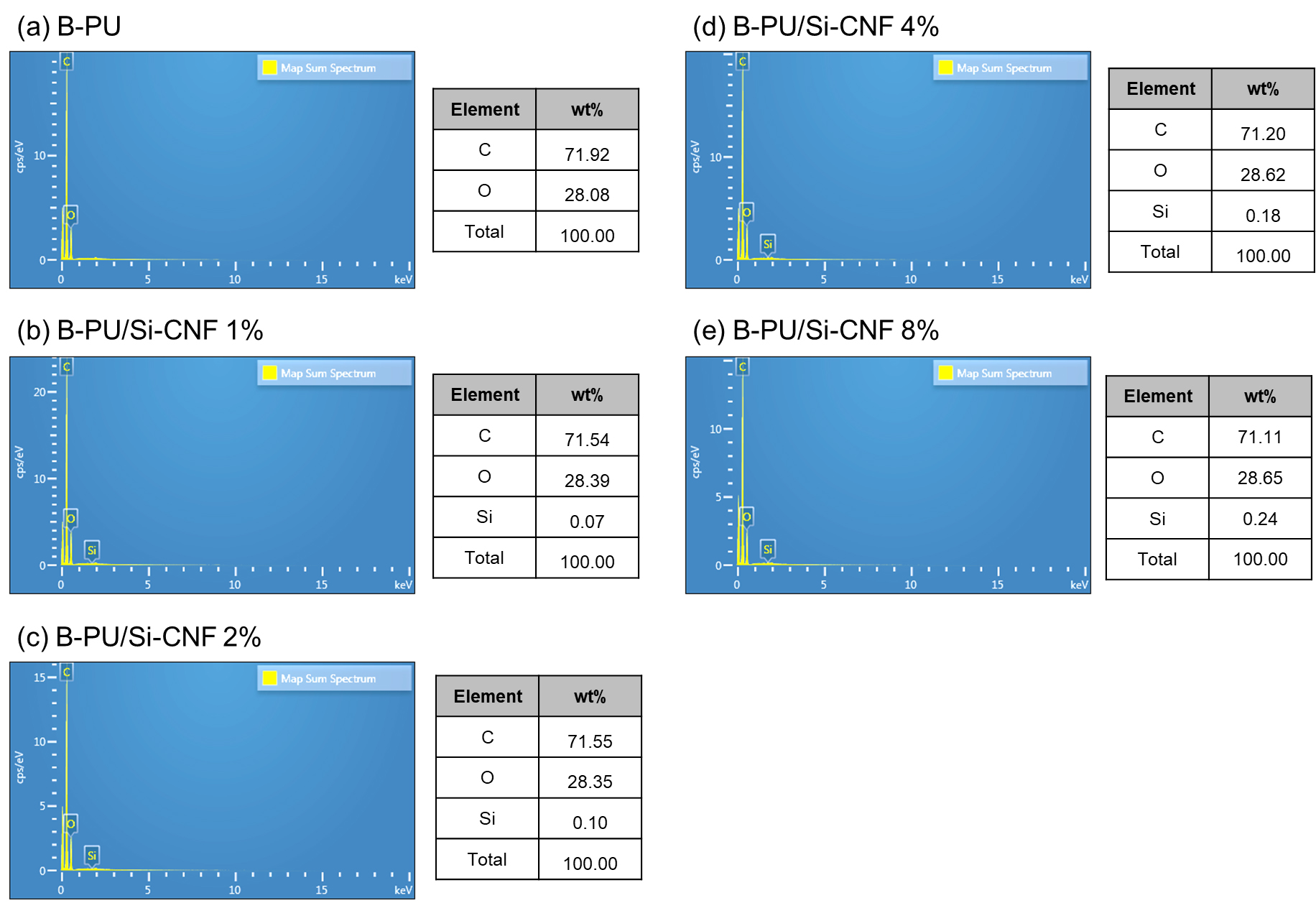
Surface analysis of pure CNF and Si-CNF was conducted using SEM (Fig. 2a, 2b), revealing a spaghetti-like nano-scale network micro-structure, including some microscale fibers. After drying, SEM showed that there were no empty spaces between the fibers, indicating close contact and good flocculation. In the case of Si-CNF, the fibrous phase was also flocculated, and it was observed that the fibrous phase took the form of MTMS conjugation, appearing rounded on the fiber. The accumulated MTMS exhibited a diameter ranging between 50 and 100 nm, and silane modification was confirmed by detecting the Si-peak during elemental analysis with EDS in Fig. 2c.
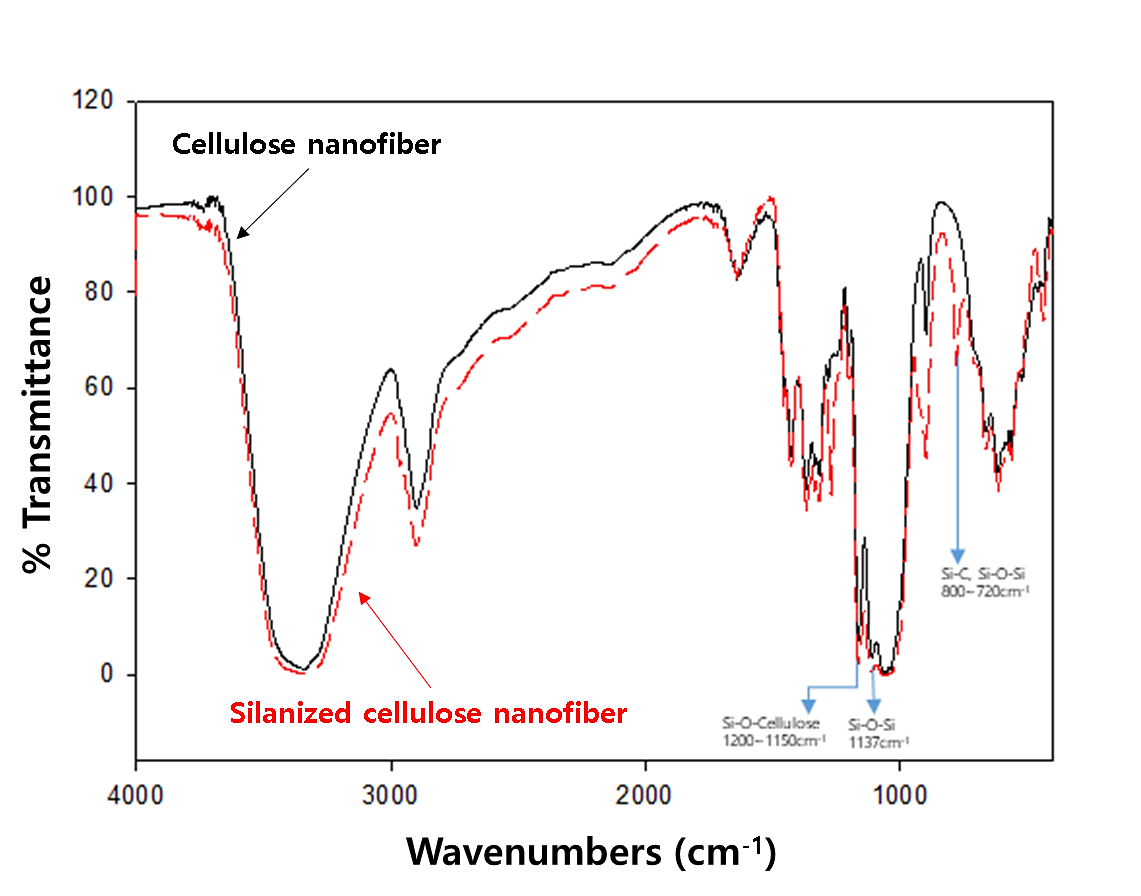
Fig. 3 illustrates the infrared spectra of CNF and Si-CNF. The primary cellulose peaks for pure CNF were observed at 1,035 cm-1 for C-O stretching, 2,900 cm-1 for C-H stretching, and 3,350 cm-1 for broad O-H stretching.28) In addition to the distinctive cellulose peaks, Si-CNFs exhibited different IR peaks, including 800–720 or 1,137 cm-1 for Si-O-Si and 800–720 cm-1 for Si-C.29,30) For instance, the FTIR data of CNF modified by MTMS, as shown in the figure, clearly demonstrate an increase in the peaks at 866 cm-1 and 1,200 cm-1 following silane alteration.
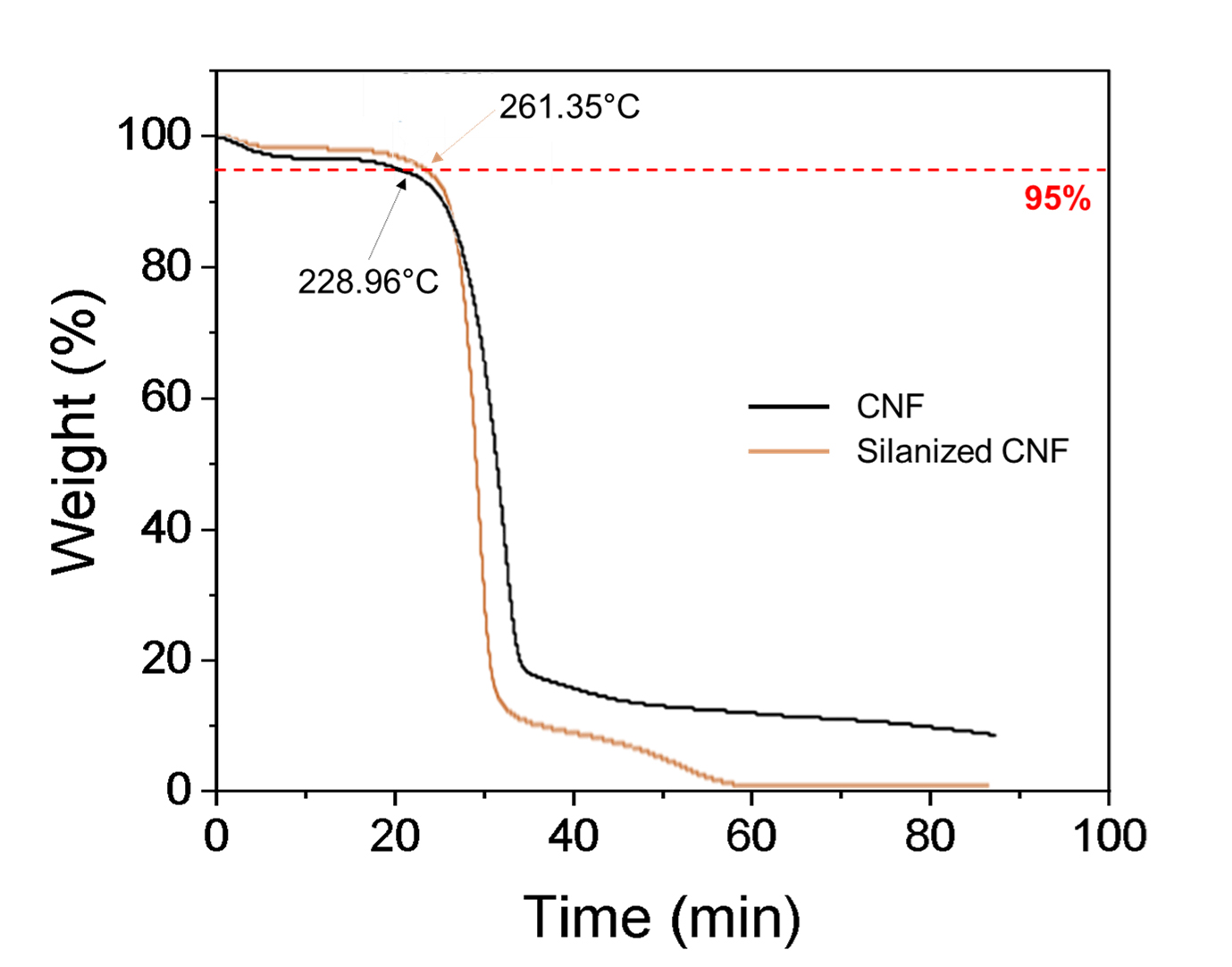
Fig. 4 is based on the pyrolysis temperature at the time of decomposition. TGA analysis was employed to determine the pyrolysis temperature of pure CNF and Si-CNF. It was confirmed that decomposition occurred at 228.96°C for untreated cellulose nanofibers, whereas for Si-CNF, decomposition started from 261.35°C with 94.9% residue remaining in both cases. The increase in pyrolysis temperature demonstrates that silane modification enhances stability against heat.
3.3 Characterization of bio-urethane/silane-modified cellulose nanofiber composites
Fig. 5 depicts a cross-section of B-PU films containing CNF and Si-CNF, examined with SEM at a magnification of 10,000 times. It is observed that the higher the silane-modified CNF content, the greater the density of Si-CNFs in the cross-section. This observation aligns with the results of EDS elemental analysis shown in Fig. 6, indicating an increasing trend in the content of Si-CNFs. Additionally, it is noted that the film surface undergoes significant changes at silane concentrations of 4% and 8%, becoming almost rough. These morphological changes can be attributed to variations in the dispersion of Si-CNF on the film surface depending on fiber concentration.
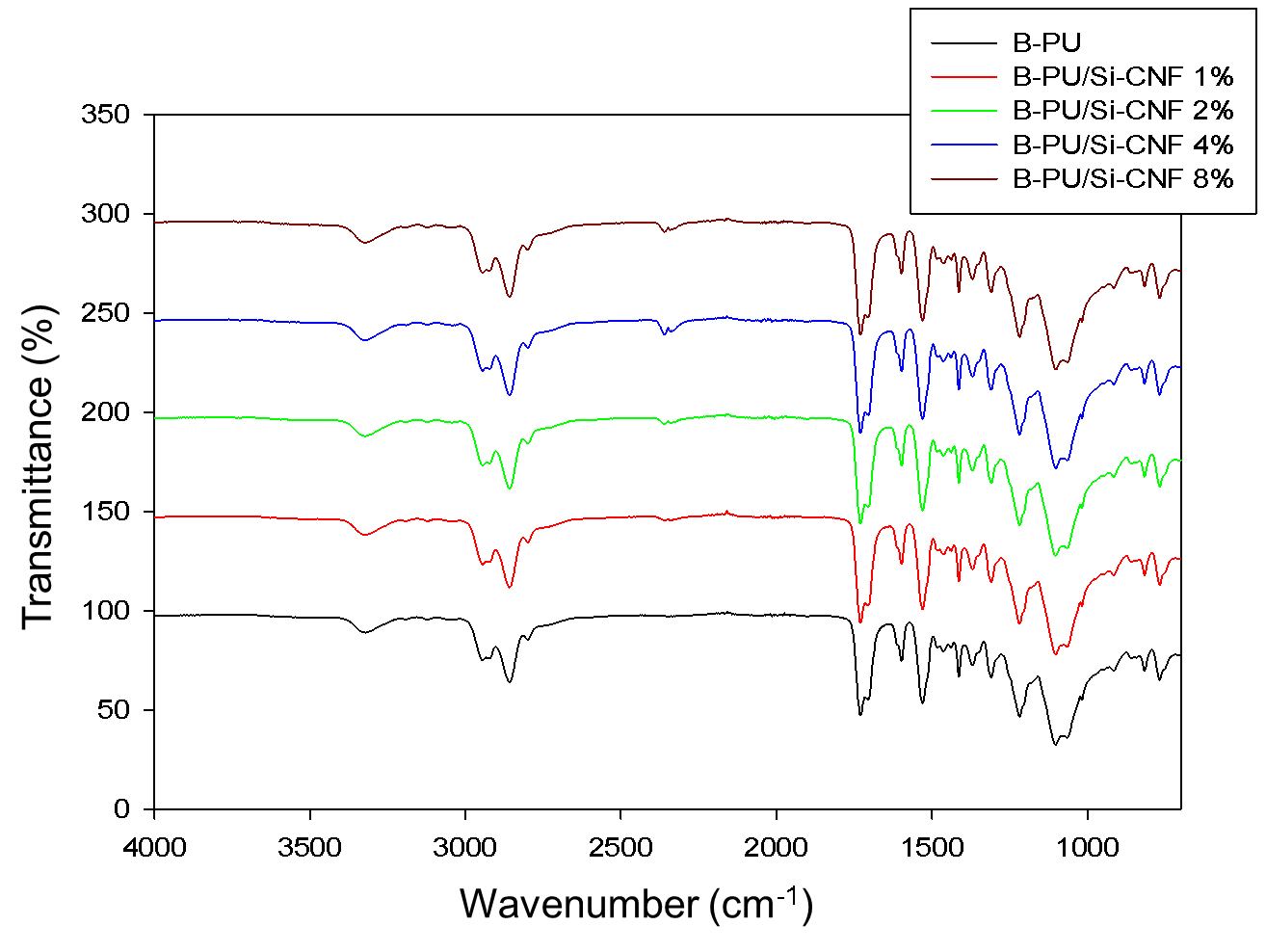
The FT-IR spectra of B-PU based composites containing various amounts of Si-CNF are presented in Fig. 7. To assess how the presence of filler affects the matrix structure, these spectra were compared with the spectrum of B-PU. The stretching vibration of the -NH bond is represented by the wavenumber 3,321 cm-1. The bending vibration of the NH bond, typical of the urethane group, is responsible for the band at 1,530.1 cm-1. The stretching vibrations of the CH2 group are represented by the wavenumbers 2,858.4 and 2,944.9 cm-1, while the deformation vibrations of the same group are represented by the wavenumber 1,437 cm-1. Both hydrogen-bonded and free carbonyl groups appear as a multiplet in a wavenumber range from 1,701 to 1,730 cm-1, whereas the stretching vibration of the C=O group occurs at 1,218.2 cm-1. The vibrations associated with a free C-O-C group and a hydrogen-bonded C-O-C group were present at the wavenumbers 1,101.9 and 1,060.11 cm-1, respectively.31) Slight changes in the intensity and peak position of these bands indicate the presence of hydrogen bonds between the matrix and the filler.
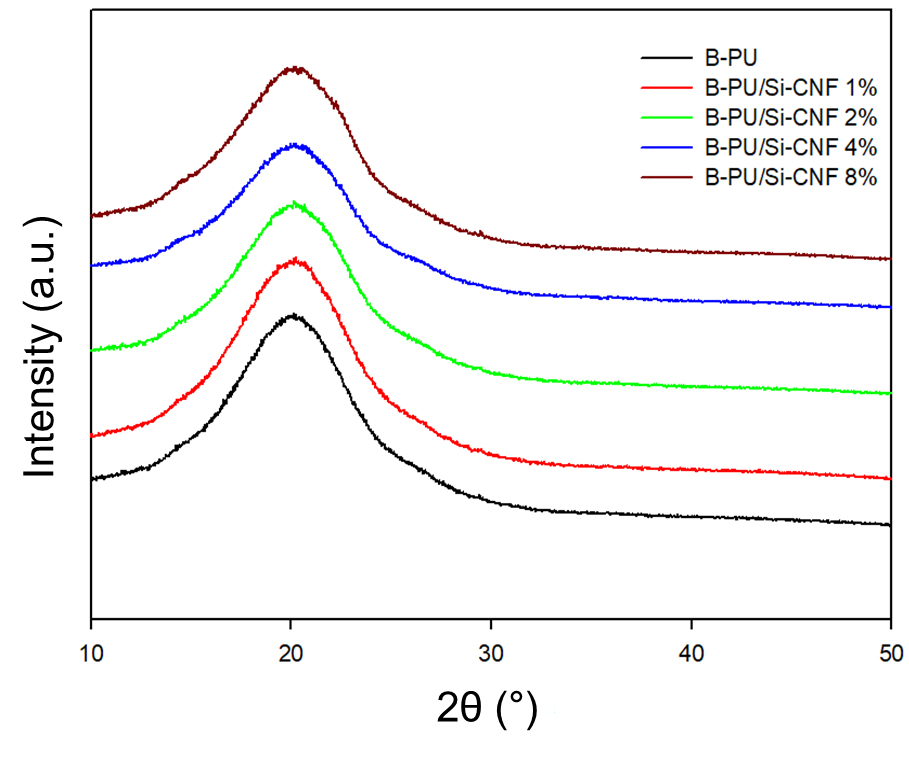
Fig. 8 displays the XRD pattern of the B-PU/Si-CNF nanocomposite film. For the neat B-PU film, a broad characteristic peak was observed at about 20°, indicating its amorphous behavior.32) No additional peaks were observed for the B-PU/Si-CNF (1–8 wt.%) nanocomposite film, except for the typical broad peak at around 20°. The 2θ values of B-PU, B-PU/Si-CNF 1 wt.%, B-PU/Si-CNF 2 wt.%, B-PU/Si-CNF 4 wt.%, B-PU/Si-CNF 8 wt.% were 20.06°, 20.15°, 20.23°, 20.24°, 20.06°, and 20.32° respectively. Due to different concentrations of Si-CNF, peaks appeared at various 2θ values. When comparing BPU samples with and without Si-CNF, it can be observed that the presence of Si-CNF promotes the development of a more ordered structure in the B-PU. Higher peak intensities of the samples (B-PU/Si-CNF 1 wt.%) suggest higher crystallinity compared to B-PU. Since BPU/Si-CNF 1 wt.% exhibits the highest peak intensity, its structure is more uniform. Additionally, the samples BPU/Si-CNF (2–8 wt.%) have lower diffraction peak heights than B-PU, indicating that they contain smaller crystal particles, reducing their crystallinity and making them more amorphous. This might result from the random distribution of Si-CNF in B-PU. Anjum et al. observed a similar behavior where the crystallinity of PU samples decreased with an increase in the filler content.33)
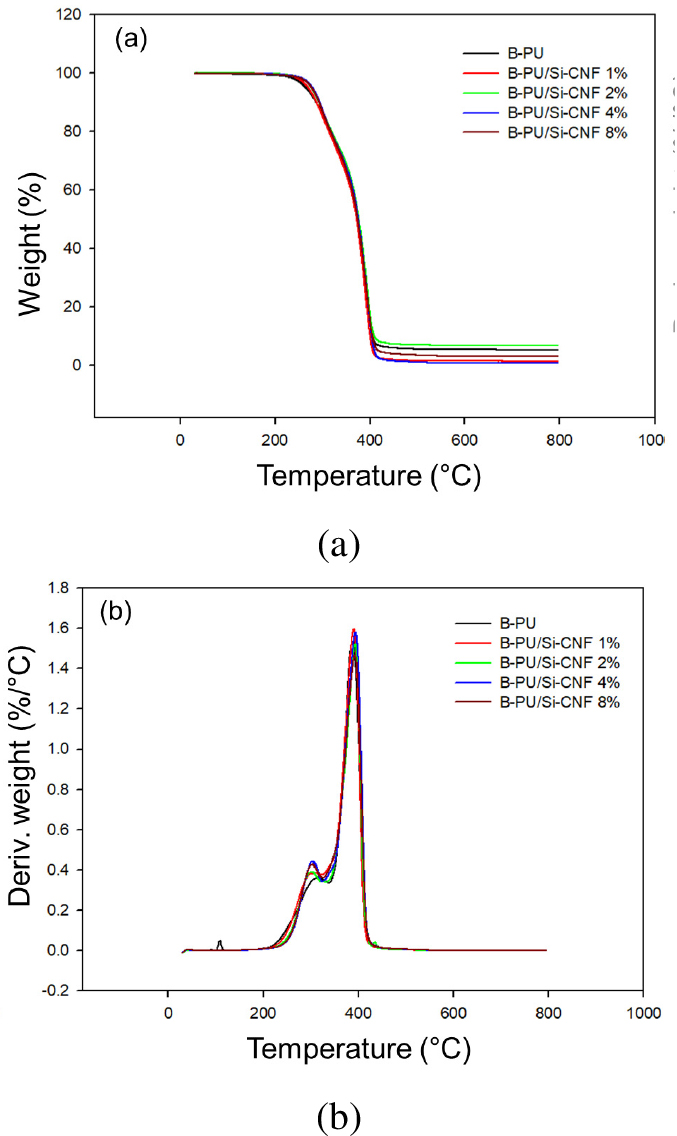
One of the objectives of adding CNF to B-PU was to evaluate its impact on the thermal stability of composites. Figs. 9a and 9b show the TGA and DTG (first derivative of the TGA) curves for pure B-PU and B-PU/Si-CNF composite films, respectively. Table 1 summarizes the thermal parameters, including T5% (the 5% weight loss temperature), Tmax (maximum degradation temperature), and maximum weight loss rate (WLRmax) respectively. Only a single degradation stage between 236 and 415°C was observed for the neat B-PU and B-PU/Si-CNF composites.
Table 1.
Thermal gravimetric analysis of bio-polyurethane based composites film
From the table, it is observed that T5% of B-PU/Si-CNF composite film increased from 260.31 to 264.55°C with the incorporation of Si-CNF (1–8 wt.%). Additionally, the composite films show slightly better thermal stability than the films with pure B-PU in terms of Tmax, possibly due to the rise in water-insoluble network structure with increasing Si-CNF content. For the neat B-PU films, Tmax was 387.82°C, and the maximum weight loss rate was 1.54% min-1. For the composite film, Tmax value increased from 387.82°C to 394.77°C with the addition of Si-CNF (1–8 wt.%), and the lowest WLRmax (1.46% min-1) was obtained for Si-CNF 8%. The thermal stability improved with the incorporation of Si-CNF into the B-PU matrix, which can be attributed to the interaction between Si-CNF and B-PU, as well as the good dispersion of Si-CNF in the B-PU matrix.
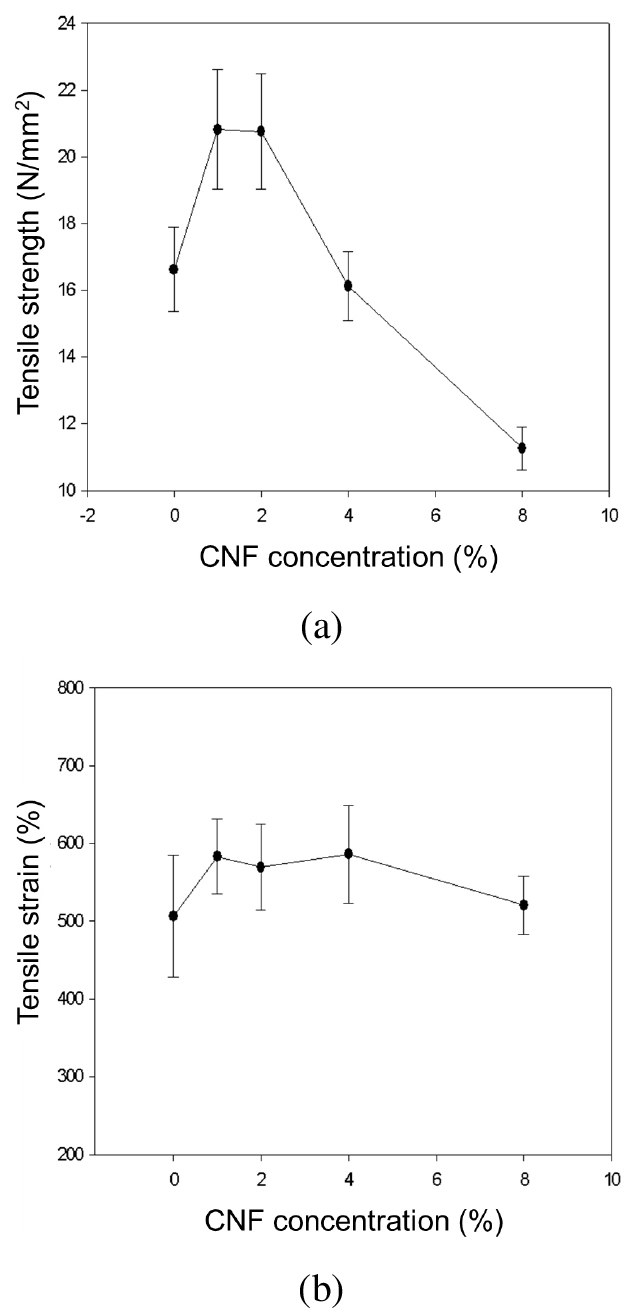
To assess whether the reinforcing effect has changed, tensile strength and tensile elongation were measured (Fig. 10). The composite film exhibited the highest tensile strength for 1% Si-CNF, maintaining a high tensile strength at 2%, but it decreased at 4%, resembling that of neat PU. At 8%, the tensile strength significantly decreased. Regarding tensile elongation, the polyurethane film containing modified CNF demonstrated a higher elongation value compared to the polyurethane film without modified CNF. It tended to increase at 4% content, with a slightly reduced elongation value at 8%, although it still exhibited a higher elongation compared to the B-PU without CNF.
4. Conclusions
In this study, environmentally friendly nanocellulose fibers were modified with MTMS to impart hydrophobic properties. SEM surface analysis confirmed the modification, and elemental analysis using EDS revealed the presence of Si peaks. FT-IR identified chemical functional groups in CNF and Si-CNF. The higher pyrolysis temperature indicated improved heat stability due to silane modification. Tensile strength tended to increase for polyurethanes with 1–2% of Si-CNF, but decreased at higher concentrations (4–8%). Elongation increased up to 4% CNF content, with a slight decrease at 8%. Compatibility issues between hydrophobic CNFs and B-PU resins may contribute to reduced tensile strength or elongation at higher content. XRD results showed B-PU/Si-CNF 1wt.% had the highest crystallinity. The composite films exhibited slightly better thermal stability than pure B-PU films, possibly due to increased water-insoluble network structure with higher Si-CNF content.