1. Introduction
2. Materials and Methods
2.1 Materials
2.2 Preparation of BC hydrogel
2.3 Preparation of a hydrogel ink containing bacteria
2.4 Preparation of 3D structured BC hydrogel
2.5 Preparation of freeze-thawed BC/GelMA composite hydrogel
2.6 Preparation of photo-crosslinked BC/GelMA composite hydrogel
2.7 Mechanical properties of BC/GelMA composite hydrogel
2.8 Cell viability assay
3. Results and Discussion
3.1 Photo-crosslinking and freeze-thawing processes
3.2 Chemical and morphological properties of composite hydrogels
3.3 Mechanical properties of composite hydrogels
3.4 Effect of sequential processes on the mechanical properties of composite hydrogels
3.5 Cell viability of the composite hydrogels
4. Conclusions
1. Introduction
Hydrogel-based materials have flexible properties due to their high water content, and many studies have attempted to use them in biomaterials.1,2) Biomedical engineering applications require materials with excellent mechanical strength, bioactivity, and a 3D structure.3,4) Since conventional hydrogel materials have insufficient mechanical reliability, it is necessary to develop a new approach to fabricating mechanically stable hydrogels without toxic chemicals.5,6)
Bacterial cellulose (BC) hydrogel is a hydrophilic polymer containing a large amount of water that forms a stable structure of a 3D network biosynthesized by bacteria.7) BC hydrogels have excellent shape stability and mechanical properties compared to other natural polymeric hydrogels even if the structure undergoes a cleaning and purification process or mechanical strain is applied.8) BC hydrogels are also bio-friendly natural polymers because they do not show cytotoxicity.4) Despite the highly stable 3D network structure of BC, its bioactivity is not good enough to support the growth and proliferation of tissue cells.4) From the previous studies, BC is usually combined with other materials like particles or suspensions after breaking the network.4) In these cases, the unique properties of BC are not retained. Meanwhile, a water-soluble molecule can penetrate into nanocellulose networks through diffusion and easily form composites with network templates.9)
Gelatin methacrylate (GelMA) has been used in biomedical engineering including bio-printing10) and cell encapsulation,11), and it was used as a diffusible bioactive molecule for interpenetration into BC networks. GelMA hydrogels have essential properties like cell-attaching arginine-glycine-aspartic acid (RGD) sequences, promoting cell growth and proliferation in GelMA-based scaffolds.12) Upon exposure to light irradiation, GelMA also crosslinks to form hydrogels with tunable mechanical properties. Hybrid hydrogel systems can be fabricated by incorporating GelMA with nanoparticles such as carbon nanotubes,13) graphene oxide,14) and other polymers to form networks with needed combined properties and characteristics. Recent research has demonstrated that GelMA-based hydrogels can be used in various biomedical applications including the engineering of skin,14) cartilage,15) and vascular tissues.16)
Here, GelMA is combined with BC to compensate for the poor bioactivity of BC through crosslinking. We try to diffuse GelMA molecules into the BC networks to promote interpenetration without destroying the original 3D structure of BC, which provides the superior mechanical strength of the composite hydrogel. In addition, a freeze-thaw process is adopted in the research to improve the mechanical properties of BC/GelMA composite hydrogel. The freeze-thaw process increases the alignment of molecules and improves the mechanical properties of hydrogels by filling the space between the nanofiber networks with densely packed polymeric fillers.17) Interestingly, the efficiency of the reinforcement is dependent on the order of the photo-crosslinking and freeze-thaw processes due to the different packing mechanisms of GelMA molecules. Such a bioactive BC/GelMA hydrogel composite with enhanced mechanical properties can be applied as biomaterials for cell and tissue engineering.
2. Materials and Methods
2.1 Materials
Gluconacetobacter xylinus (G. xylinus, KACC 17012) was obtained from the Korean Rural Development Administration. The bacteria were cultured on an HS medium prepared with glucose (Duksan science), yeast extract (Becton, Dickinson and Company, Franklin Lakes, NJ), bacto peptone (Becton, Dickinson and Company), sodium phosphate dibasic 12 hydrate (Junsei Chemical), and citric acid monohydrate (Showa Chemical Industry Co.). Polytetrafluoroethylene (PTFE) microparticles (TF 1641, 3 M Dyneon) were used as a hydrophobic solid matrix for 3D printing. GelMA powder was purchased from 3D materials. Lithium phenyl(2,4,6-trimethyl benzoyl) phosphinate (LAP) was purchased from Tokyo Chemical Industry. Phosphate-buffered saline (1× PBS solution, pH 7.4) was purchased from Welgene (Gyeongsan, Korea).
2.2 Preparation of BC hydrogel
The culture medium was prepared by adding 2.0% (w/w) of glucose, 0.5% (w/w) of yeast extract, 0.5% (w/w) of Bacto Peptone, 0.27% (w/w) of sodium phosphate dibasic dodecahydrate and 0.12% (w/w) of citric acid monohydrate to distilled water (Kim et al. 2022). The culture medium was sterilized by autoclaving at 120°C for 20 min. The culture medium was inoculated with G. xylinus and stirred for 30 min. The culture medium containing G. xylinus was filtered with a mesh filter to remove residues during the inoculation. The culture medium was incubated in a chamber for 7 days at room temperature. The pure BC hydrogel was biosynthesized on the surface in plate form. The pure BC hydrogels were immersed in 1% NaOH solution for 24 h and then washed in flowing distilled water for 24 h. The pure BC hydrogels were stored in PBS at 4°C until they were used.
2.3 Preparation of a hydrogel ink containing bacteria
A hydrogel ink was prepared by mixing 1.25% (w/w) carboxymethylated cellulose nanofiber, 2.0% (w/w) of glucose, 0.5% (w/w) of yeast extract, 0.5% (w/w) of Bacto Peptone, 0.27% (w/w) of sodium phosphate dibasic hydrate and 0.12% (w/w) of citric acid monohydrate. The mixed hydrogel ink and HS medium was sterilized by autoclaving at 120°C for 20 min. The hydrogel medium ink was then inoculated with G. xylinus and stirred for 30 min. The culture medium containing G. xylinus was filtered with a mesh filter to remove residues during the inoculation.18)
2.4 Preparation of 3D structured BC hydrogel
3D structured BC hydrogels were biosynthesized with the hydrogel ink containing bacteria printed in the PTFE particle matrix. The bacteria were incubated in a chamber for 7 days at room temperature. The biosynthesized 3D structured BC hydrogels were immersed in 1% NaOH solution for 24 h and then washed in flowing distilled water for 24 h. The prepared 3D structured BC hydrogels were stored in PBS at 4°C until they were used.18)
2.5 Preparation of freeze-thawed BC/GelMA composite hydrogel
The GelMA powder was dissolved in water at 37°C for 15 min. The solution was heated in a bath under 37°C for 15 min. 3D structured BC hydrogels were immersed in a 3% GelMA solution for 24 h. The BC templates that had absorbed GelMA were taken out of the GelMA solution, subsequently frozen at –15°C for 22 h, and then thawed at room temperature for another 6 h. The freeze-thaw cycle was repeated 3 times. The freeze-thawed BC/GelMA composite hydrogels were stored in PBS at 4°C until they were used.
2.6 Preparation of photo-crosslinked BC/GelMA composite hydrogel
The GelMA powder was dissolved in water at 37°C for 15 min. 3D structured BC hydrogels were immersed in a 3% GelMA solution for 24 h and in a 1 mM lithium phenyl-2,4,6-trimethyl-benzoylphosphinate solution for 1 h. The UV irradiation was repeated twice for 2 min. The photo-crosslinked BC/GelMA composite hydrogels were stored in PBS at 4°C until they were used.
2.7 Mechanical properties of BC/GelMA composite hydrogel
The tensile strengths of the BC/GelMA composite hydrogel were measured using a universal testing machine (GB/LRX Plus, Lloyd, West Sussex, UK) fitted with a 500 N load cell at a strain speed of 1.00 mm/s. The BC/GelMA composite hydrogel was cut into specimens with a thickness of 2.5 mm, length of 40 mm, and width of 10 mm. The tensile stress was set to the break strain, and five specimens were tested for each hydrogel. The compressive strength was evaluated using a universal test machine fitted with a 500 N load cell at a strain speed of 2.00 mm/min. The BC/GelMA composite hydrogel was cut into cylindrically shaped specimens with a diameter of 8 mm and a height of 2.5 mm. The compressive test was set to 95% strain, and five specimens were tested for each hydrogel. The BC/GelMA composite hydrogel was prepared with a thickness of 5 mm. All tests were performed at 50% humidity and 25°C.
2.8 Cell viability assay
NIH 3T3 fibroblast and HeLa cells were cultured in Dulbecco's Modified Eagle's medium (DMEM, Invitrogen, San Diego, CA, USA) supplemented with 10% fetal bovine serum (Life Technologies, NY, USA) and 1% penicillin/streptomycin (Life Technologies) at 37°C and 5% CO2. Cells were maintained in tissue culture polystyrene at a confluency of 70%. Pure BC and BC/GelMA composite hydrogels were soaked in DMEM at 37°C for one day. Fibroblast and HeLa cells were added to each hydrogel substrate and cultured for 7 days. The viability of the cells was investigated with a live and dead viability/cytotoxicity kit (Invitrogen, Waltham, MA, USA) following the protocol (incubation for 1h at room temperature in the dark). The fluorescence images of the cells were collected using a fluorescence microscope (Logosbio Celena S Digital Microscope, Anyang, Republic of Korea). To investigate cell proliferation, a petri dish containing cells was washed once with PBS and incubated with alamarBlue® (Invitrogen) solution for 4 h at 37°C. The fluorescence intensity was assayed at 570 nm using a microplate reader (Synergy HT, BioTek, Winnoski, USA).9)
3. Results and Discussion
3.1 Photo-crosslinking and freeze-thawing processes
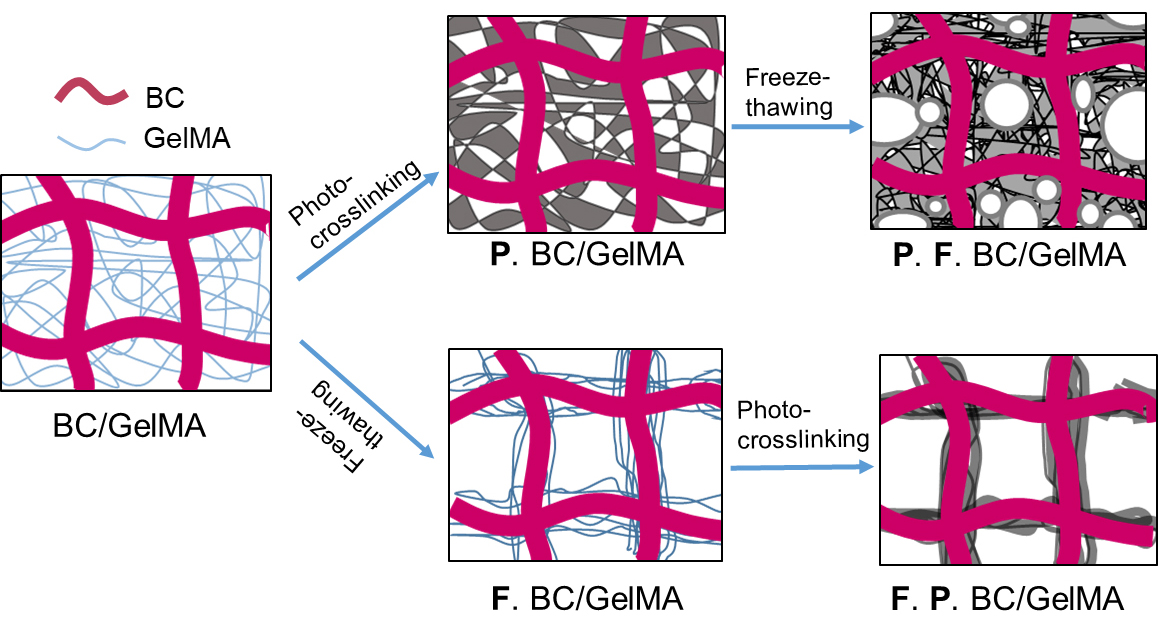
Photo-crosslinking and freeze-thawing processes were performed to create a composite hydrogel with the excellent mechanical properties of BC and the excellent bioactivity of GelMA. A tubular form of BC was biosynthesized from the surface of printed hydrogel ink by the embedded G. xylinus, an aerobic bacterium. The BC tubes were composited with GelMA by the diffusion method. BC was immersed into the GelMA solution to diffuse the GelMA between 3D networks of BC. Photo-crosslinking, which is a type of chemical crosslinking, was performed through UV irradiation and freeze-thawing, which is a type of physical crosslinking, was achieved through a repetitive process. After going through the crosslinking steps, a BC/GelMA composite hydrogel with a double network structure was formed.
To investigate the effect of GelMA crosslinking on the network structure, five different samples were prepared including one control and four composite hydrogels using different crosslinking strategies: 1) the non-crosslinked BC/GelMA hydrogel in which GelMA was simply diffused into BC networks, 2) photo-crosslinked BC/GelMA hydrogel without freeze-thawing (P. BC/GelMA), 3) photo-crosslinked BC/GelMA hydrogel followed by freeze-thawing (P. F. BC/GelMA), 4) freeze-thawed BC/GelMA hydrogel without photo-crosslinking (F. BC/GelMA) and 5) freeze-thawed BC/GelMA hydrogel followed by photo-crosslinking (F. P. BC/GelMA) (Fig. 1).
Photo-crosslinking results in a GelMA network as chemical crosslinking occurred (P. BC/GelMA). The freeze-thawing of P. BC/GelMA pushes the GelMA network through the growth of ice crystals, resulting in the accumulation of networks within the BC network (P. F. BC/GelMA). Freeze-thawed BC/GelMA (F. BC/GelMA) forms a physically crosslinked network as GelMA chains are pushed out by ice crystals. GelMA chains align along the BC network structures during this process. The photo-crosslinking of F. BC/GelMA produces a GelMA network between the pushed GelMA chains along the BC network by chemical crosslinking (F. P. BC/GelMA).
3.2 Chemical and morphological properties of composite hydrogels
FT-IR spectra of pure BC, pure GelMA, and BC/GelMA composite hydrogels were obtained to analyze the chemical composition of the composite hydrogels (Fig. 2a). FT-IR spectra showed the peaks at 3341 cm-1, 2940 cm-1, and 1030 cm-1, which were assigned for –OH stretching, alkyl C-H, and C-O-C stretching, respectively. GelMA showed peaks at 3320 cm-1, 1657 cm-1, and 1536 cm-1, which were assigned for N-H stretching, C=O stretching, and N-H deformation, respectively. The BC/GelMA composite showed typical peaks for BC and GelMA in the spectrum, confirming the successful formation of a composite of the two materials.
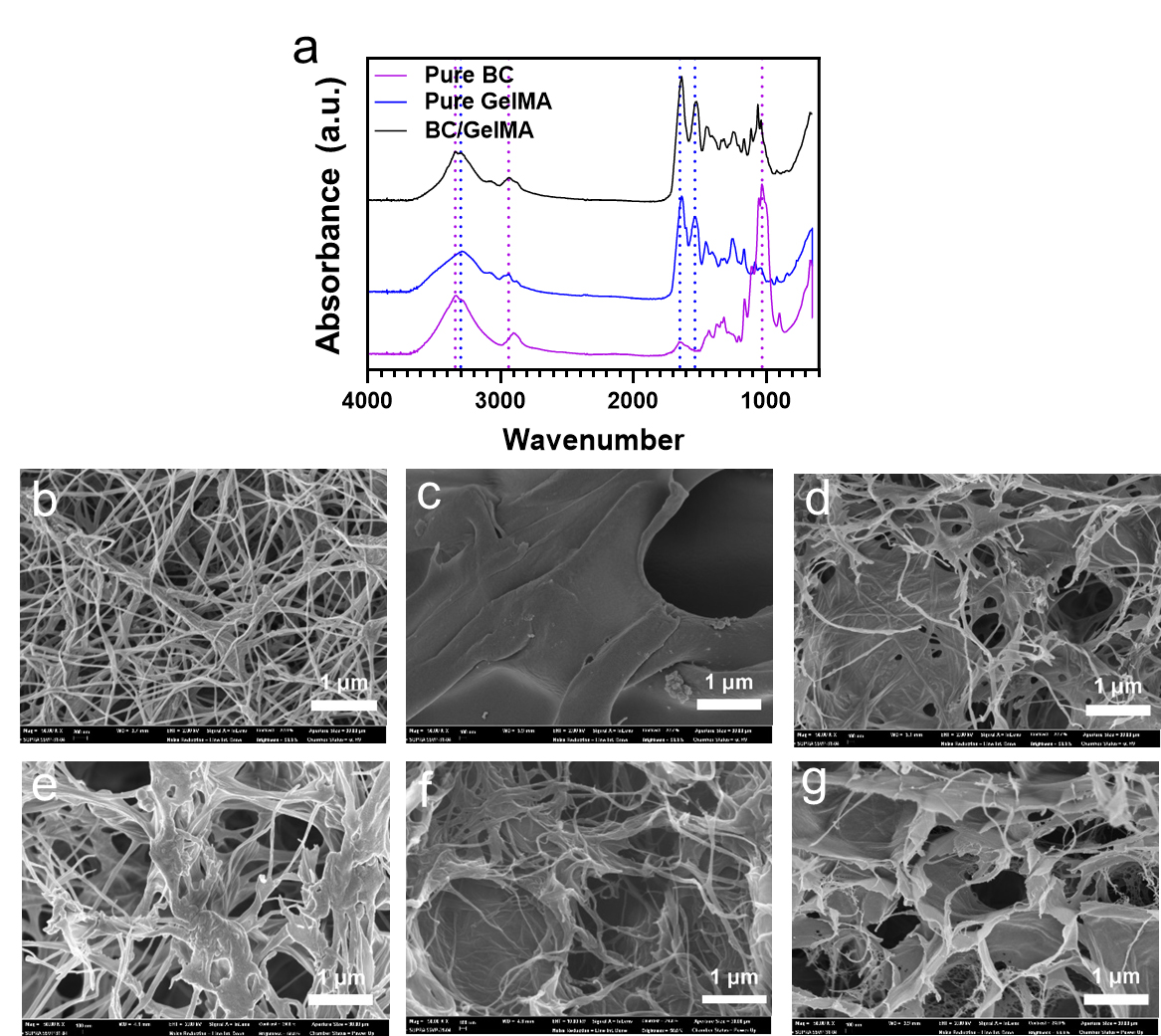
Fig. 2.
(a) FT-IR spectra of pure BC, pure GelMA, and BC/GelMA composite hydrogels. Cross-sectional SEM images of (b) pure BC hydrogel, (c) pure GelMA hydrogel, (d) photo-crosslinked BC/GelMA composite hydrogel without freeze-thawing (P. BC/G), (e) freeze-thawed BC/GelMA composite hydrogel without photo-crosslinking (F. BC/G), (f) freeze-thawed BC/GelMA composite hydrogel followed by photo-crosslinking (F. P. BC/G), and (g) photo-crosslinked BC/GelMA composite hydrogel followed by freeze-thawing (P. F. BC/G).
Nanofibers were observed using an SEM for BC, while the plate-like structure was observed in a photo-crosslinked GelMA image (Fig. 2b and 2c). The photo-crosslinked BC/GelMA hydrogel showed both nanofibers and plates (Fig. 2d). The freeze-thawed BC/GelMA composite hydrogel without photo-crosslinking showed the original porous BC structures and GelMA molecules bound around the BC nanofibers (Fig. 2e). Some of the GelMA molecules could be removed during the cleaning process due to weak binding. The freeze-thawed BC/GelMA followed by photo-crosslinking showed a plate-like structure in addition to the nanofibers (Fig. 2f). The porous structures were made during the freeze-thaw process and the nanofibers moved due to the growth of ice crystals (Fig. 2f). In contrast, the photo-crosslinked BC/GelMA showed a porous plate-like structure rather than the porous nano-fibric structure (Fig. 2g). This is because the photo-crosslinking created a plate-like structure at the first step, and the plates formed pore walls due to the growth of ice crystals in the freeze-thawing process.
3.3 Mechanical properties of composite hydrogels
Tensile and compressive tests were performed with pure BC and BC/GelMA composite hydrogels to investigate the mechanical properties of the composite hydrogels. We compared the ultimate stress, elongation at break, Young's modulus, and toughness. First, the effect of GelMA concentration on the mechanical properties was investigated (Fig. 3). According to the results, mechanical properties in ultimate tensile strength (Fig. 3a), Young’s modulus (Fig. 3c), toughness (Fig. 3d), compressive strength (Fig. 3e), and compressive modulus (Fig. 3f) tend to increase as the GelMA concentration increases. However, the elongation at break (Fig. 3b) tends to decrease.
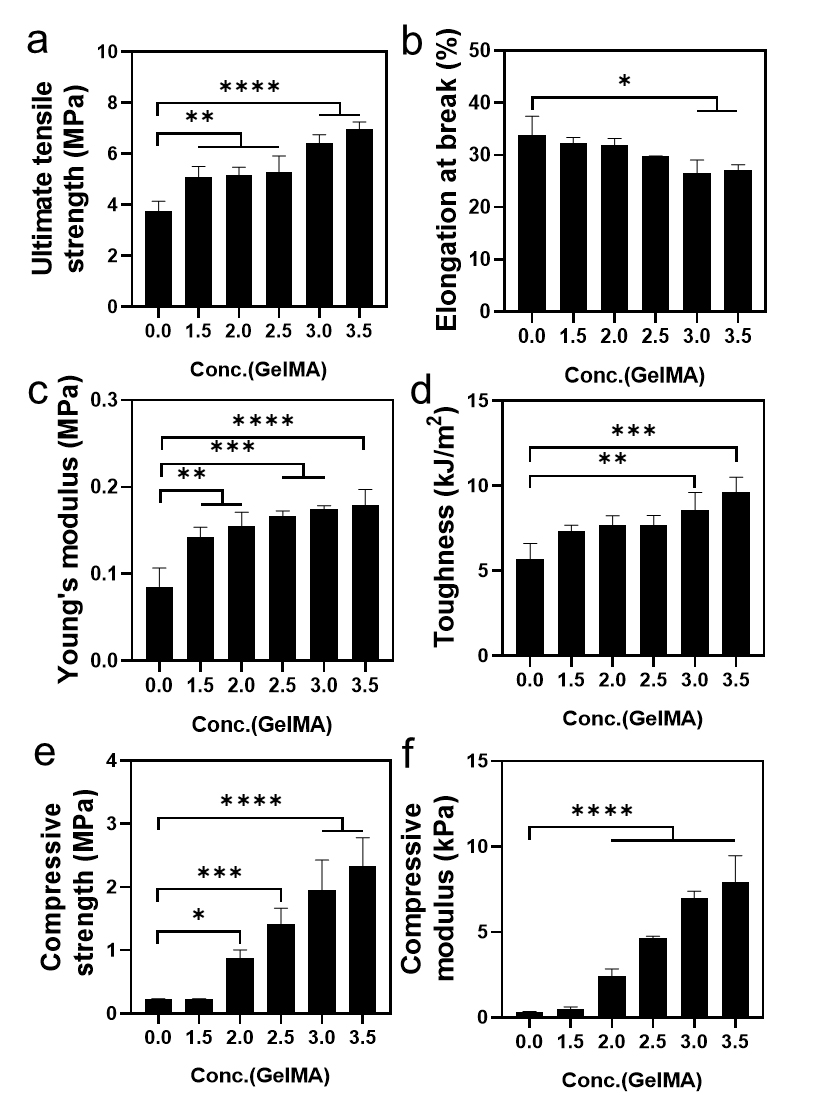
Fig. 3.
Mechanical properties of BC/GelMA composite hydrogel as a function of GelMA concentration. (a) Ultimate tensile strength, (b) elongation at break (c) Young’s modulus, (d) toughness, (e) compressive strength, and (f) compressive modulus. BC/GelMA composite hydrogels were prepared by photo-crosslinking.
In the BC/GelMA composite hydrogel, GelMAs formed a double networked structure with the BC structure. Since the network density of the GelMA increased as the GelMA concentration increased, the mechanical properties of the BC/GelMA composite improved, allowing it to withstand higher tensile and compressive stresses (Fig. 3a). However, the mobility of the polymer chain inside the composite hydrogel decreased because of the increase in the chemical crosslinking point. The reduced chain mobility decreased the flexibility of the BC/GelMA composite hydrogel. Thus, the elongation at break decreased as the GelMA concentration increased (Fig. 3b). Meanwhile, the tensile stress increased significantly as the GelMA concentration increased, and the Young’s modulus showed an increase with increasing GelMA concentration (Fig. 3c). The toughness of BC/GelMA composites increased as the GelMA concentration increased due to the significant increase in tensile stress especially at 3% and 3.5% of GelMA concentrations (Fig. 3d). Similar to the tensile test, the compressive properties also showed an increase in compressive strength and modulus, (Fig. 3e and 3f).
3.4 Effect of sequential processes on the mechanical properties of composite hydrogels
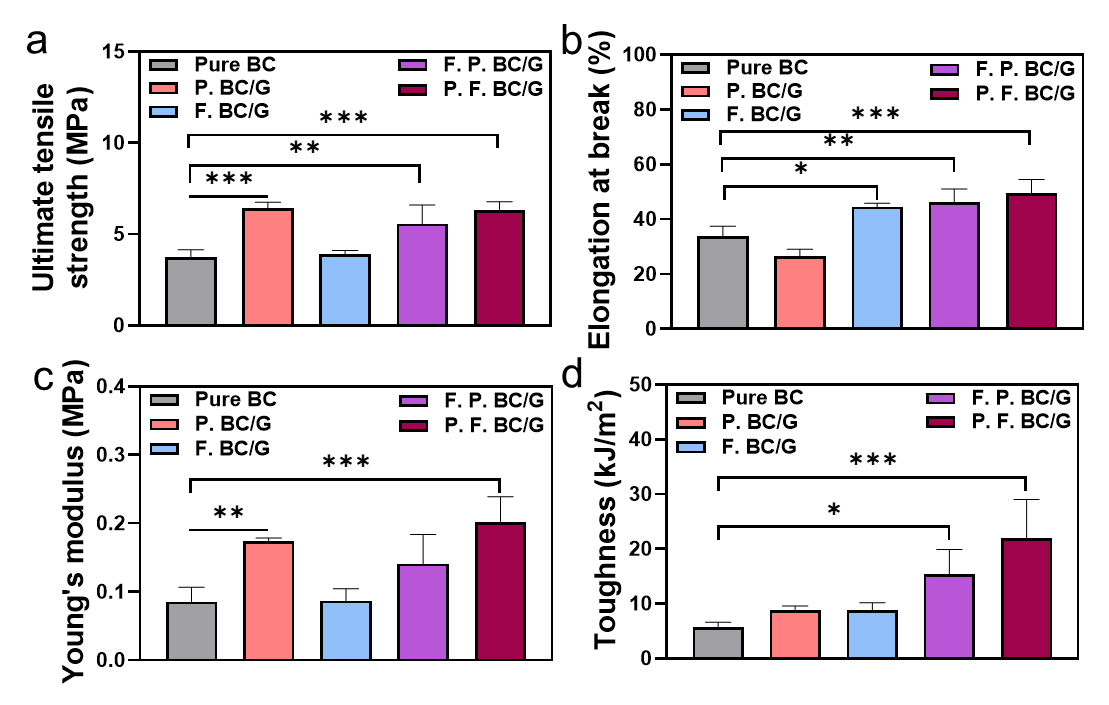
It was interesting that the two different crosslinking processes provided similar toughnesses of composite hydrogels, which is the combined result of the stress and strain. Despite the increase in the crosslinking or network points, the contribution to the mechanical properties can vary depending on the order of the process.
F. P. BC/GelMA first undergoes a freeze-thaw process to reduce the distance between the GelMA molecules in the BC network. The locally concentrated GelMA around BC nanofibers resulted in insufficient UV exposure, and the GelMA with few crosslinking points or no crosslinks can be removed during the washing-out process. In contrast, P. F. BC/GelMA showed a relatively higher ultimate tensile strength (Fig. 4a), elongation at break (Fig. 4b), Young’s modulus (Fig. 4c), and toughness (Fig. 4d).
The sequential process of double-network formation is crucial for the significant enhancement of the mechanical properties of the composite hydrogels. Moreover, chemical crosslinking by photo-crosslinking contributes to the increase in mechanical properties.
In the freeze-thaw process GelMA aligned along the BC nanofibers and dissipated the applied energy through the networks, increasing the value of elongation at the break. In addition, the ultimate tensile strength also increased sequentially due to the highly networked structure caused by photo-crosslinking. Because the effect of strengthening the mechanical properties was greater than the factors described above that reduce strength, the ultimate tensile strength, elongation at the break, and toughness values increased.
In the photo-crosslinking process, GelMA formed a dense layer (like a wall between the BC nanofibers), and the ultimate tensile strength and Young’s modulus values increased due to the high network structure. The elongation at break also increased sequentially due to the energy dissipation of the physical network formed through a freeze-thaw process.
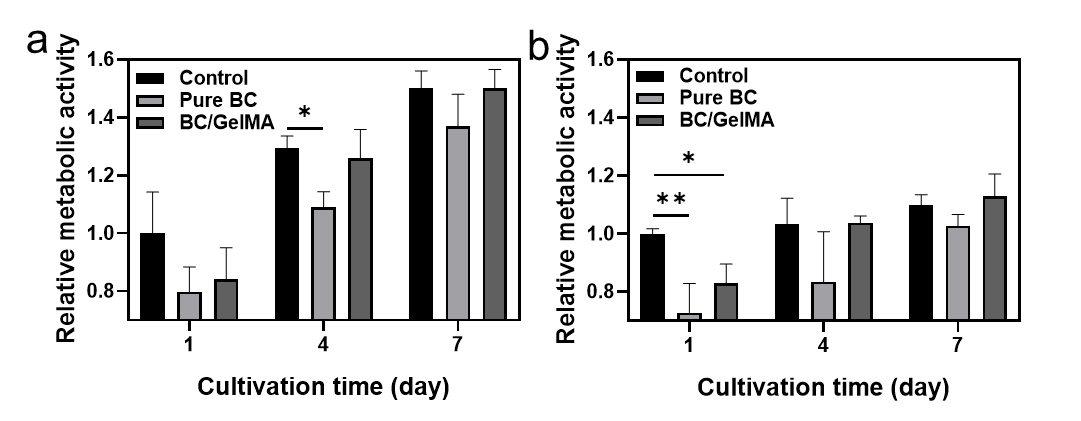
3.5 Cell viability of the composite hydrogels
Cell growth and proliferation are critical for the application of BC/GelMA composite hydrogels in cell and tissue engineering. Metabolic activity for the NIH 3T3 cell and HeLa cells was examined using an alamarBlue assay, and the BC/GelMA composite hydrogels induced a higher bioactivity compared with the pure BC, confirming their biocompatibility for use in biomedical engineering (Fig. 5). The assay of NIH 3T3 cells confirmed that the cell viability with the composite hydrogel was similar to the control surface or higher than that of the pure BC hydrogel (Fig. 5a). In addition, the incubation of HeLa cells also confirmed the high cell viability with the composite hydrogels (Fig. 5b).
4. Conclusions
BC and GelMA were combined to realize synergistic improvements in mechanical properties and biocompatibility compared to pure BC and GelMA hydrogels. The 3D networked BC was interpenetrated with GelMA, which formed new double networks through crosslinking reactions. In addition, a freeze-thaw process was introduced to increase the mechanical properties of composite hydrogels by packing the BC nanofibers and GelMA molecules through the growth of ice crystals. Interestingly, the different types of crosslinking processes provided some different hydrogel properties. The combined process of freeze-thawing and photo-crosslinking effectively improved the mechanical properties of the BC/GelMA composite hydrogel compared to the other BC/GelMA composite hydrogels. In particular, the photo-crosslinked BC/GelMA followed by the freeze-thawing was superior in tensile strength and toughness compared to the pure BC. In terms of biocompatibility, BC/GelMA also showed higher metabolic activity compared to pure BC. Overall, the tough BC/GelMA composite hydrogels could be used in biomedical engineering research in the future.