1. Introduction
2. Materials and Methods
2.1 Materials
2.2 Methods
3. Results and Discussion
3.1 Measurement of the degree of substitution of commercial carboxymethylcellulose with 3 different methods
3.2 Effect of chemical concentration on degree of substitution of carboxymethylation of cellulose
3.3 Effect of reaction temperature and time to degree of substitution of carboxymethylation of cellulose
4. Conclusions
1. Introduction
Nanocellulose is cellulose that has been processed to have a width of several to tens of nanometers and a length of hundreds to thousands of nanometers through physical and chemical treatment. It is classified into cellulose nanofibrils (CNF) by physical treatment and cellulose nanocrystal (CNC) by chemical acid hydrolysis, and is also made of bacterial cellulose (BC).1,2) Nanocellulose is known to have a larger specific surface area and aspect ratio than cellulose, higher strength and modulus, high modulus of elasticity, high modulus of elasticity, high modulus of 150-250 GPa, and high strength of more than 5 GPa.3,4,5) It is superior to the physical properties of carbon and glass fiber, and it has advantages such as low coefficient of thermal expansion, light weight, dimensional stability, and eco-friendliness, so research is underway to apply it to electronic materials, adsorbents, and reinforced fillers.6,7,8,9) The mechanical processing apparatus for manufacturing cellulose nanofibrils exerts a strong force on the cellulose to cut the amorphous region.10,11,12) The device mainly uses a grinder-type equipment with ultra-precise abrasive discs and a high-pressure homogenizer method that sprays cellulose under high pressure through a narrow tube. Manufacturing cellulose nanofibrils by physical treatment is less harmful to the environment but requires more energy than cellulose nanocrystals using acids and bacterial cellulose using microorganisms. To break the hydrogen bonds in cellulose, the plant must be treated dozens of times, consuming more than 27,000 kWh of energy per ton of cellulose.13)
To solve the problem of energy consumption in the manufacture of cellulose nanofibrils, research has been carried out on physical and chemical pretreatment. The physical method is to cut the cellulose using a beater or refiner used in the pulp and paper industry. Chemical pretreatment methods are chemical reforming methods in which cellulose is introduced with an anion or cation so that it has electrical properties.14) The TEMPO-oxidation method is a method of oxidizing the position of carbon 6 of cellulose to a carboxyl group, which makes it electrical properties. The other method is to use the conventional method of preparing carboxymethyl cellulose, which introduces a carboxymethyl group into the cellulose so that it has electrical properties similar to that of TEMPO-oxide cellulose.15,16)
It has been reported that the application of enzymes, carboxymethylation, and TEMPO-oxidation pretreatment in the cellulose nanofibril manufacturing process can be reduced to 500-1,500 kWh per ton. In addition to the energy-saving effect of the cellulose nanofibril manufacturing process through pretreatment, the physical properties of cellulose nanofibril are different. In particular, it has been reported that the cellulose nanofibril width is reduced in the process of chemical pretreatment such as carboxymethylation and TEMPO-oxidation.13)
The physical properties such as the length and width of the prepared cellulose nanofibril vary depending on the conditions and degree of carboxymethylation pretreatment, but there has been no research on the degree of pretreatment so far.
Therefore, in this study, we wanted to study the carboxymethylation during chemical pretreatment, and to confirm the degrees of carboxymethylation according to the conditions. The carboxymethylation reaction was performed on the commercially available bleached hardwood Kraft pulp, and the different degree of substitution and substitution position according to the carboxymethylation reaction condition were checked by the variables of sodium hydroxide, acetic acid chloride, temperature, and time among the carboxymethylation reaction conditions. In order to measure the degree of substitution of carboxymethylation, the degree of substitution of the carboxymethylated pulp prepared under conditions was measured using titration method, infrared spectroscopy, and nuclear magnetic resonance spectroscopy (NMR), and the location of the carboxymethyl group was confirmed through nuclear magnetic resonance spectroscopy analysis.
2. Materials and Methods
2.1 Materials
The pulp used for carboxymethylation was bleached hardwood Kraft pulp provided by Moorim P&P. Sodium hydroxide (96.0%, OCI), acetic acid chloride (98.0% Samjeon Chem), and ethanol (95% Samjeon Chem.) were used for the carboxymethylation substitution and substitution location analysis, and sulfuric acid (96.0%, Samjeon Chem.), 30% hydrochloric acid (Samjeon Chem.), and 1% phenolphthalein solution (Samjeon Chem.) was used for the analysis of carboxymethylation substitution and substitution location. Carboxymethylcellulose with substitution degrees of 0.7, 0.9, and 1.2 was purchased and used from Sigma-Aldrich to confirm the accuracy of the substitution measurement method.
2.2 Methods
2.2.1 Preparation of carboxymethylation pretreatment of bleached hardwood Kraft pulp
The amount of chemical addition and reaction conditions for carboxymethylation are shown in Table 1. The reaction sequence was to dissociate 10.0 g of pulp in 200 ml of ethanol dissolved in sodium hydroxide and leave it at room temperature for 2 hours to produce negative ions in the pulp. After mixing 150 ml of ethanol dissolved in chloride acetic acid in the pulp left at room temperature, the pulp was carboxymethylated according to the temperature and reaction time. After the reaction was completed, the pulp was neutralized and washed to pH 6-7 using acetic acid and distilled water after removing the ethanol and used for the analysis of the degree of substitution and the location of the substitution.
Table 1.
Reaction conditions for carboxymethylation of LBKP
2.2.2 Analytical methods
2.2.2.1 Carboxylate in carboxymethylated pulp by phenolphthalein titration.
Mix 2.0 g of carboxymethylated pulp with 200 mL of distilled water and 50 mL of 0.3 N sodium hydroxide solution in a 500 mL beaker and stir for 5 hours at room temperature to prepare a suspension. After adding 2-3 drops of 1% phenolphthalein solution to the carboxymethylated pulp suspension, titration by the end point of phenolphthalein solution was performed using a 0.3 N hydrochloric acid solution. By measuring the volume of the consumed 0.3 N hydrochloric acid solution, the degree of substitution of the consumed 0.3 N hydrochloric acid solution, and the degree of substitution of the carboxymethyl group by the Eq. 1 and Eq. 2.
Where, B: mol of NaOH normality of NaOH in initial titration
C: mol of HCl normality of HCl in used titration
D: gram of carboxymethylated pulp
2.2.2.2 Carboxylate in carboxymethylated pulp by FT-IR spectroscopy
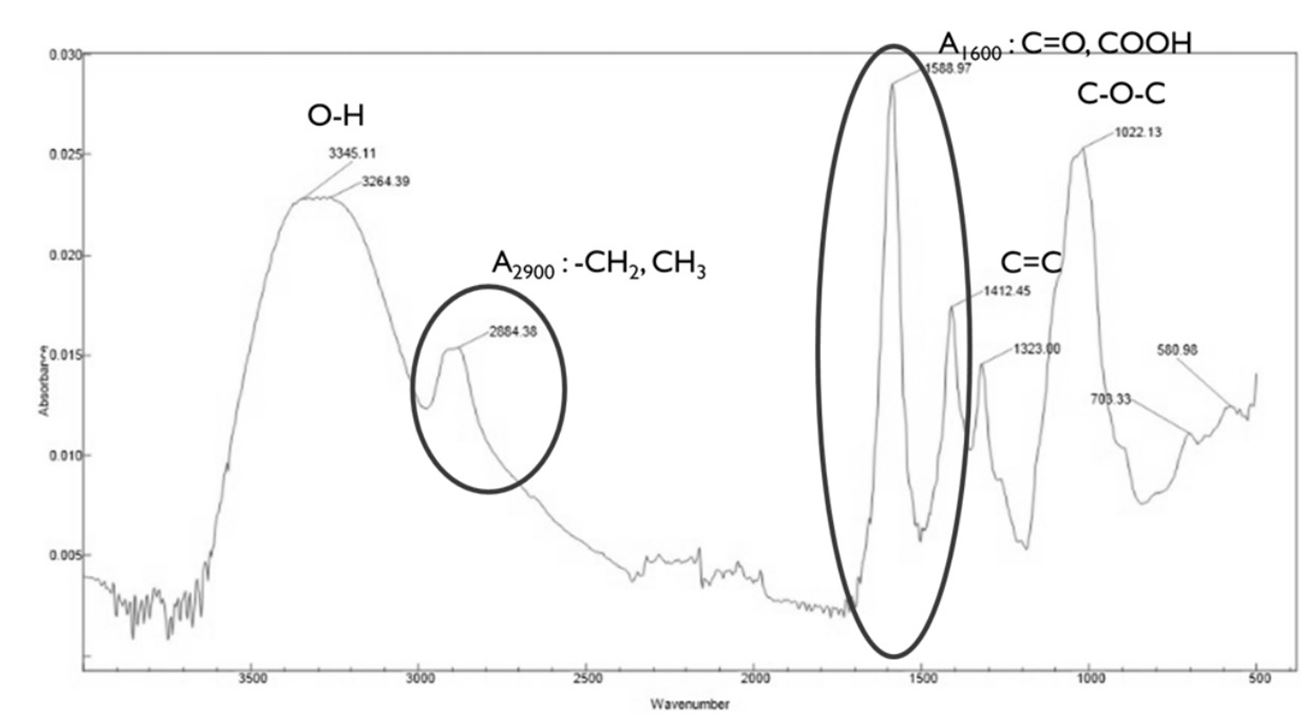
To analyze the carboxymethyl substitution of bleached hardwood Kraft pulp, we used the FT-IR (Fourier Transform Infrared Spectrometer; Cary670, Agilent, USA) to perform infrared spectroscopic analysis. All samples were measured in powder form, and the measured wavelength range was 400-4,000 cm–1.17) The measured spectra were analyzed as shown in Fig. 1 and the degree of substitution was analyzed using the following Eq. 3.
Where, A: absorbance of carboxyl group (-COOH)
B: absorbance of methyl group (-CH2)
2.2.2.3 Carboxylate in carboxymethylated pulp and location of substitution by NMR spectroscopy
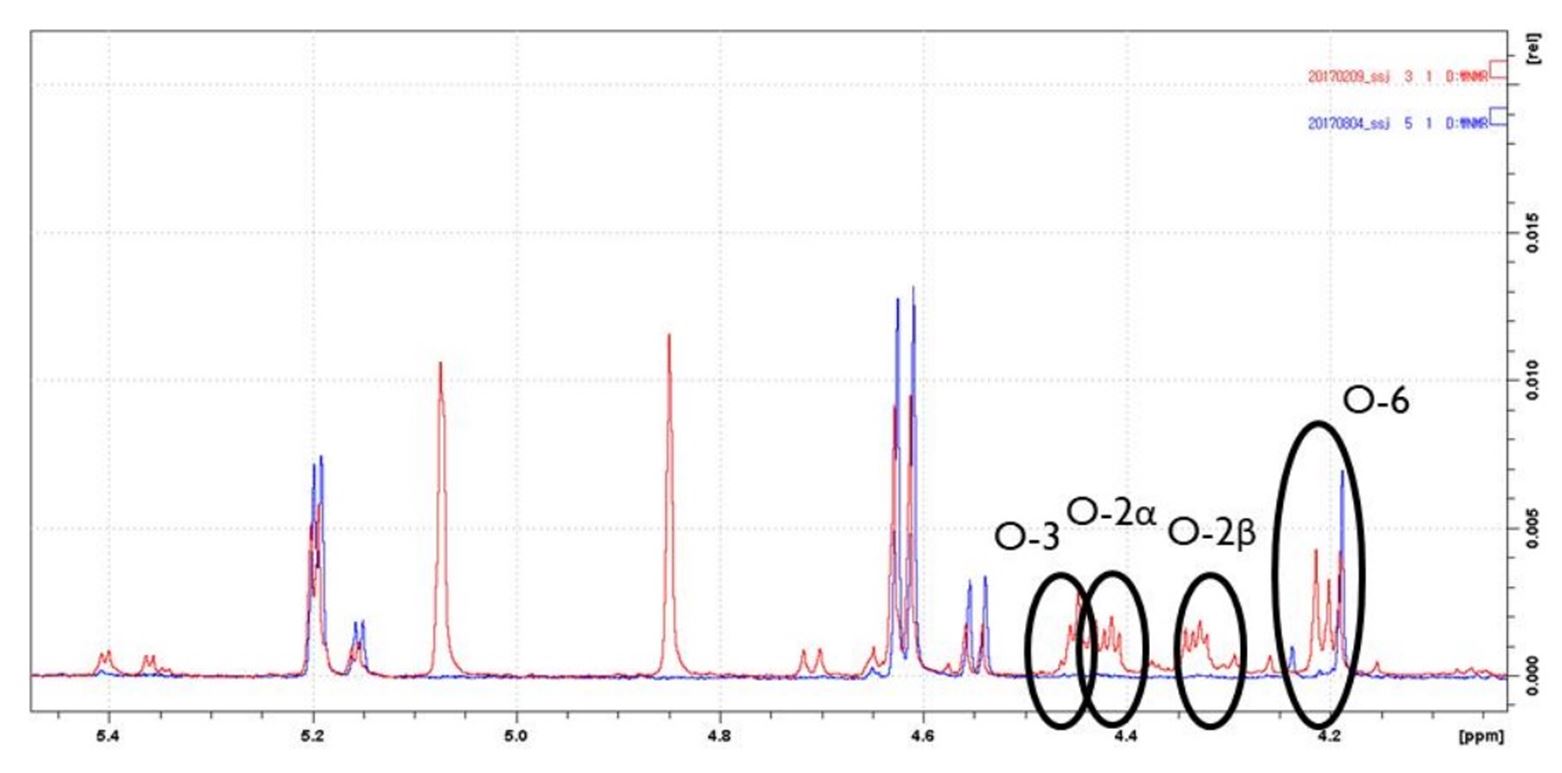
The 1H-NMR analyzer was used to analyze the degree of substitution and substitution position of carboxymethylated pulp. After adding 40 mg of sample to the pressure tube, 0.6 mL of 72.0% sulfuric acid was added and the primary hydrolysis was performed in the shaker at 30℃ for 1 hour. After the first hydrolysis, the acid was diluted by adding 3.0 mL of heavy water (D2O), and the second hydrolysis was performed in an oven at 100℃ for 1 hour. After the reaction was completed, the filtrate was collected after filtering with quantitative filter paper, and 1H-NMR analysis was performed after adding 7 drops of concentrated sulfuric acid.18) The instrument was a Bruker AVACE NMR spectrometer (500 MHz), and the data were quantitatively and qualitatively analyzed by integrating the anomeric hydrogen peak and the carboxyl group peak on the spectrum as shown in Fig. 2 using the Topspin program. The integral values were calculated using the following Eq. 4 and Eq. 5 to calculate the displacement of bleached kraft pulp of carboxymethylated hardwoods.19)
Where, A: peak of methylene protons at position O-i (i=2,3,6)
B: peak of anomeric protons at position H-1α (O-2s)
C: peak of anomeric protons at position H-1α (O-2u)
D: peak of anomeric protons at position H-1β (O-2s)
E: peak of anomeric protons at position H-1β (O-2u)
3. Results and Discussion
3.1 Measurement of the degree of substitution of commercial carboxymethylcellulose with 3 different methods
The results of the substitution of commercially available carboxymethylcellulose of 0.7, 0.9, and 1.2 are measured by phenolphthalein titration, infrared spectroscopy, and nuclear magnetic resonance spectroscopy as shown in Table 2. Except DS 0.9, actual DS were 0.1 higher than marked in commercial products. Phenolphthalein titration is a method of measuring the content of carboxylmethyl groups introduced by the carboxymethylation reaction by acid-base neutralization titration. Carboxylic acids are weak acids with a pKa value of 4.5 and are mostly in ionized form. The neutralization reaction begins to proceed with the addition of acid, and since the equilibrium of this reaction is slow, the neutralization reaction must be carried out carefully and slowly to determine the correct neutralization point. Therefore, it takes a long time to measure the titration point through the neutralization reaction. Not only that, but it also discolors rapidly at the neutralization point, so you have to operate slowly near the neutralization point to find the correct neutralization point.
Table 2.
Degree of substitution of carboxymethyl cellulose by means phenolphthalein titration FT-IR and 1H-NMR spectroscopy
| Sample | Degree of substitution | ||
| Phenolphthalein titration | FT-IR | NMR | |
| CMC 0.7 | 0.83±0.04 | 0.80±0.01 | 0.84±0.02 |
| CMC 0.9 | 0.91±0.03 | 0.92±0.02 | 0.90±0.01 |
| CMC 1.2 | 1.36±0.04 | 1.38±0.04 | 1.33±0.01 |
The substitution measurement method by infrared spectroscopy irradiates the pulp with a carboxymethyl group and analyzes the spectrum according to the degree of energy absorption corresponding to the vibration and rotation of the molecular skeleton. By analyzing the absorbance of CH2 and COOH positions in the spectrum, CH2 is judged to be glucose, and COOH is judged to be the substituted carboxymethyl group to calculate the degree of substitution. Since the amount of glucose is calculated as CH2 in the process of calculating the substitution of carboxymethyl groups, the greater the number of molecules without CH2 in the molecule, the greater the substitution of the carboxymethyl group.
The substitution measurement method by nuclear magnetic resonance spectroscopy decomposes a sample with a carboxymethyl group substituted into glucose and then performs nuclear magnetic resonance analysis to obtain the spectrum. In the obtained spectrum, the substitution is calculated by integrating the position of the anomeric hydrogen and the position of the carboxymethyl group. By analyzing the sample after hydrolysis, it is necessary to manipulate the sample so that it is accurately degraded to confirm the correct.
As a result of the measurements, the three methods obtained similar results, but the DS 0.7 and 1.2 samples obtained higher results than the DS suggested in the reagents. Therefore, the substitution of carboxymethylcellulose for which the substitution was provided was measured as about 0.8, 0.9, and 1.3 instead of 0.7, 0.9, and 1.2. As a result of comparing the three methods of measuring the degree of substitution, the error of the phenolphthalein titration method was the largest, and the margin of error was the smallest in the hydrogen nuclear magnetic resonance spectroscopy method. Phenolphthalein titration is thought to be due to incomplete titration due to the time it takes to reach the equilibrium of weak acids, and infrared spectroscopy should be compared as a baseline, but errors are thought to occur due to the difficulty of setting a standard baseline.
3.2 Effect of chemical concentration on degree of substitution of carboxymethylation of cellulose
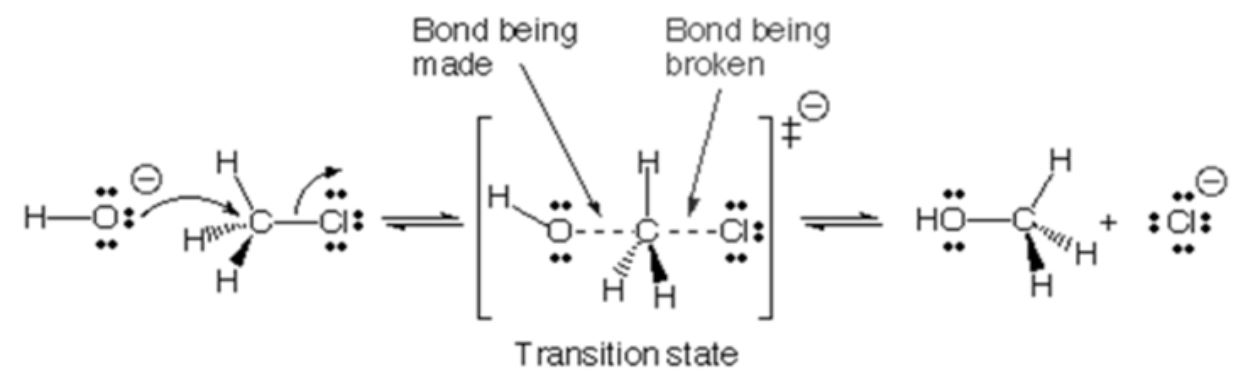
The change in carboxymethyl substitution according to sodium hydroxide content during the carboxymethylation reaction is shown in Table 3. The substitution of carboxymethyl groups according to the sodium hydroxide content increased when the concentration of acetic chloride that could react with the abrasiveness of the anion produced by sodium hydroxide increased (experimental conditions 1, 2, and 3), but the substitution did not increase when an excess of acetate chloride (case 4) was used. The change in carboxymethyl substitution according to chlorinated acetic acid content in the carboxymethylation reaction is shown in Table 4. In the carboxymethylation reaction, the anionized cellulose attacks acetic chloride, causing chlorine to dissociate, and the carboxymethyl group makes the cellulose equivalent. The carboxymethylation reaction was thought to be an SN2 reaction, as shown in Fig. 3, as it depends on both the ionization of alcohol by sodium hydroxide and the concentration of acetic chloride. Cellulose is ionized into an anion by sodium hydroxide dissolved in ethanol, and this anion acts as a nucleophile as shown in Fig. 4. The anionic nucleophile produced causes acetic acid chloride to undergo an SN2 reaction, and a carboxymethyl group is substituted for cellulose, and sodium chloride (NaCl) is produced as a by-product. If sodium hydroxide does not produce enough nucleophiles, the carboxymethyl group will not be sufficiently replaced during the reaction time, even though there is enough acetic acid chloride. Therefore, for an effective carboxymethylation reaction, a quantitative reaction of sodium hydroxide and acetate chloride is required.
Table 3.
Degree of substitution of carboxymethylated pulp prepared by different sodium hydroxide condition
Table 4.
Degree of substitution of carboxymethylation pulp prepared by different monochloroacetic acid condition
3.3 Effect of reaction temperature and time to degree of substitution of carboxymethylation of cellulose
The change in carboxymethylation substitution with reaction temperature is shown in Table 5. When the chloroacetic acid content was constant and sodium hydroxide was added to make one nucleophilic body per cellulose, the substitution was negligible in the reaction at a reaction temperature of 25℃, 72 hours. Sodium hydroxide content was added to produce 3 negative ions per cellulose, and a reaction of 25% of the target substitution was achieved in a 72-hour reaction at 25℃. When the temperature was increased to 70℃ with the same amount of chemical added, the target substitution was reached in the 3-hour reaction.
The substitution degree differs according to the sodium hydroxide content at low temperatures, and although the substitution reaction occurs under low temperatures, it is presumed that the substitution reaction does not occur in all three substituents of cellulose, but only in a part of it at low temperatures. Chemical reactions occur at low temperatures with a higher degree of stabilization (thermodynamic control), and at high temperatures, reactions with a faster reaction speed (reaction rate control) occur. In chemical reactions, the conditions under which the reaction occurs at low temperatures (low energy) can be selectively made because the chemical reaction is determined by the temperature. However, the conditions under which reactions occur at high temperatures (high energy) are those that have sufficient energy, so the reaction speed begins with the reaction speed and competes with the chemical reactions that occur at low temperatures.
The results of the substitution location analysis using nuclear magnetic resonance spectroscopy are shown in Table 5, and when the reaction is performed at a low temperature (sample 10), substitution occurred mainly in the hydroxyl group of carbon 6. It is assumed that the substitution reaction that occurs at carbon position 6 is a thermodynamic control reaction that occurs at low temperatures.20) It is presumed that the substitution reaction did not occur at low temperatures because the energy required for substitution was high in the 2nd and 3rd burnt positions. When the same sodium hydroxide and acetic chloride content was used and the reaction temperature was increased, the substitution positions occurred not only in 6 but also in 2 and 3 as shown in Table 6 and Fig. 5. This means that there is enough energy for the substitution reaction to occur at the 2nd and 3rd carbon sites.
Table 5.
Degree of substitution of carboxymethylated pulp prepared by different reaction times and temperatures
Table 6.
Substitution position analysis of carboxymethylcellulose and carboxymethylated pulp
4. Conclusions
In this study, the changes in the substitution of carboxymethyl groups and the substitution position by the carboxymethylation condition were analyzed. The method of measurement of carboxymethyl group substitution was measured using phenolphthalein solution titration, infrared spectroscopy, and nuclear magnetic resonance spectroscopy. To check the accuracy of each measurement method, the substitution was measured by three substitution methods with carboxymethylcellulose, and the substitution was measured in all three methods. However, the substitution measurement method by the phenolphthalein solution titration method showed a large margin of error due to its limitations.
As a result of analyzing the substitution according to the content of sodium hydroxide and acetic chloride used in the carboxymethylation reaction, the carboxymethylation reaction was confirmed to be an SN2 reaction affected by the content of sodium hydroxide and acetic acid chloride. The carboxymethylation reaction must first be carried out by the formation of cellulose anions by sodium hydroxide, and then the reaction occurs with the substitution of acetic acid chloride. Therefore, the anions of cellulose must be sufficiently produced by sodium hydroxide, and the degree of substitution is determined by the substituted acetic acid chloride.
The change in carboxymethylation substitution with temperature and time was found to match the amount of sodium hydroxide and acetic chloride added at a reaction temperature of 70℃. However, at 25℃, the substitution was found to be less than 25% of the target substitution degree. It is speculated that the substitution at lower temperatures does not occur at all positions of carbon 2, 3, and 6, but only at some locations. Among the methods for measuring the degree of substitution, if the degree of substitution is measured using nuclear magnetic resonance spectroscopy, the degree of substitution of the carboxymethyl group substitution position can be measured. Using this, the analysis of carboxymethyl group substitution locations according to carboxymethylation temperature and time showed that the reaction occurred at low temperature in carbon 6, competitive reaction occurred at carbon positions 2, 3, and 6 at high temperature, and substitution was mainly made at carbon positions 2 and 6.